
The following figure is presented here at reduced scale (resolution)to accommodate a browser. A larger scale version is available in Chapters 1 and 17 in the Download Files area that can be reached from the Site navigation bar.
All figures in this document are based on equal photon flux density per unit wavelength and not on equal energy per unit wavelength.
The scientific literature of vision has been plagued for a long time by a profusion of inconsistent spectral responses without a framework in which to understand their role. Many of them have originated from attempts to relate the absorption spectra of the chromogens called retinenes (including retinol, retinal and retinoic acid) to the spectral responses of human vision obtained by the psychophysics community. The actual chromophores of vision are the Rhodonines, not the retinenes. This subject area is discussed in detail in Chapter 5.
The absorption spectra of the Rhodonines lead directly to the observed spectral responses measured by the psychophysicist.
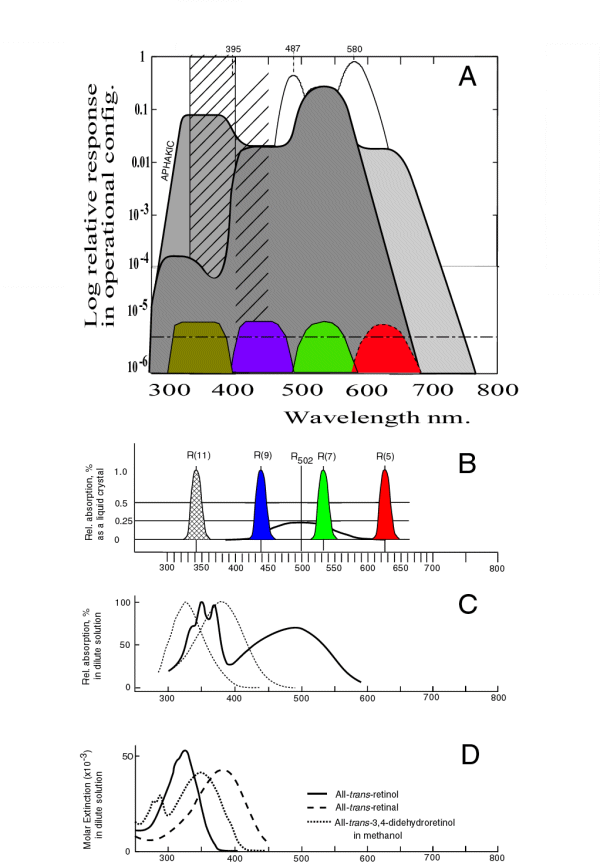
The following figure assembles all of the spectral responses related to these spectra and presents them in an ordered manner using the same linear horizontal scale. Starting from the bottom, the absorption spectra of the retinenes are provided in frame D. This data is followed by some measured data in C from one of the Rhodonines to provide continuity. Frame B provides the theoretical spectral absorption of all four of the Rhodonine chromophores using a linear amplitude scale. Frame A provides a summary of the perceived spectral responses of human vision using a logarithmic amplitude scale. To aid in understanding, the lower part of this frame includes the separately normalized absorption spectra of the Rhodonines when present in their operational configuration in the disks. The little known fact that the human eye is sensitive to radiation in the ultraviolet is discussed below and in Chapter 17 [Section 17.2].
Although much of the spectral data relevant to vision has been collected on an equal energy per unit wavelength basis, this is confusing when discussing wide spectral bandwidth systems like vision. The chromophores of vision are flux sensitive, not energy sensitive. They are photon counters. The appropriate stimulus is defined in terms of photons per unit wavelength interval. While frames A and B are based on energy measurements, frames C and D of the following figure are calculated based on photon flux units.
This frame shows the absolute absorption spectra of the three retinenes most frequently discussed in terms of vision. The solid curve represents the absorption of retinol. The conventional wisdom within the vision community considers (inappropriately) a chromophore of vision. The dotted line represents the absorption of retinal, the aldehyde of retinol. The dashed line represents retinoic acid, a slightly more complex retinene from the perspective of absorption. These materials are typical of molecules exhibiting xxx absorption as discussed in Section 5.3.4.
The peak in the absorption of retinol occurs at approximately 325 nm both in-vivo and when in dilute solution in ethanol. This is a common region for the peak absorption of organic alcohols. It is not exclusive to retinol.
The peak in the absorption of retinal occurs at approximately 357 nm in-vivo. Many values appear in the literature for the peak absorption in dilute solution in ethanol. Similarly, this is a common region for the peak absorption of organic aldehydes. It is a characteristic of the CHO radical.
The peak in the absorption of retinoic acid occurs at approximately 350 nm when in dilute solution in ethanol.
These materials do not exhibit significant absorption within the spectral range of human vision.
There have been uncountable attempts to ascribe absorption within the human visual range to these materials based on straining the form of the molecule and/or its connection to another molecule. The putative rhodopsin of the common wisdom can be described as a secondary amide of retinol and a protein. They would be formed by a reductive amination to form what is known as a Schiff-base. The Schiff-base has one proton associated with the nitrogen. Wald & Hubbard also explored an Oxime of retinol. It had a double bond between the nitrogen and carbon 15 of retinol. No visual band spectrum could be found in the literature for either of these compounds produced from laboratory reagents. Nor could a spectrum be found for a retinol-lysine-amide. [Section 5.5.3]The Rhodonines are derivable from retinol by substituion of oxygen for one of the methyl groups or a hydrogen so as to maintain a conjugated relationship between the two oxygens. Such a configuration is known as a carboxyl-ion system. This arrangement exhibits simultaneously the absorption properties expected of an alcohol, an aldehyde and an acid. However, the energy levels of the molecule are changes slightly due to the conjugation.
This frame shows a Rhodonine, on a relative scale along with retinol and retinal, the dotted curves, from Frame D. The Rhodonine curve is more complex than either of the retinenes because the molecule is more complex. It contains two oxygen atoms separated by a conjugated series of carbon atoms. The molecule shows a complex absorption profile in the ultraviolet that shows a familial relationship to the individual peaks associated with retinol, retinal and retinoic acid. The central peak is at the same wavelength as retinoic acid, 350 nm. The other peak and shoulder are near the individual wavelengths for retinal and retinol. However, the peaks are slightly closer together than either of these cases.
A single Rhodonine will simultaneously pass many simple chemical tests for alcohols, aldehydes and carboxyls.
This molecular structure also exhibits three additional absorption characteristics not shared by the retinenes. The Rhodonines consist of two heavy atoms, and a ring structure, separated by a relatively rigid carbon backbone. Because of this condition, the molecules exhibit a structural resonance that results in a bulk or molecular absorption. This is a relatively low energy resonance. It gives rise to the absorption peak at a nominal 502 nm in a dilute solution of ethanol.
The absorption characterisics discussed so far are all isotropic when measured in dilute solution.
There is another absorption mode of carboxyl-ion systems such as the Rhodonines. It is little known outside of the photographic chemistry community but has been described in theoretical detail. As the concentration of the material in Frame C is increased, a new absorption peak will appear. This peak is not observable in truly dilute solution. However, as the concentration is increased, these materials begin to form liquid crystalline strings within the solvent. These liquid crystals begin to exhibit a resonance absorption unrelated to the above molecular absorption.
Resonance absorption, or quantum-mechanical resonance absorption, is a characteristic of all high efficiency organic absorbers of visual light. It is related to the velocity of electrons traveling along the backbone of the carbonyl-ion system. The relatively low speed of this motion makes the molecule appear larger to photon radiation applied parallel to the conjugated oxygen axis of the molecule. As a result, resonance absorption occurs at wavelengths determined by the conjugation level between the two oxygen atoms. This absorption is highly anisotropic. In the Rhodonines, the peak absorption wavelength is poorly defined. The shape of the absorption characterisitic is not determined by Gaussian statistics but by Fermi-Dirac statistics. As a result, the best estimate of the peak absorption is determined by the half-amplitude points of the characteristic. In the Rhodonines, the calculated peaks obtained by averaging these two values can be given as 342, 437, 532 and 625 nm +/-2 nm.
Finally, the Rhodonines also belong to the family of indicators, similar to phenolphthalein. Therefore, their observed color in solution is a function of the pH of the solution. Many investigators have been unaware of this property and failed to control the pH of their experiments. These materials can be bleached by merely changing the pH of the solvent, such bleaching does not require external light.
If one raises the concentration in a solution of individual Rhodonines in ethanol or other solvent, they will form additional liquid crystalline strings within the solvent or precipitate out on appropriate substrates. The molecules within the strings or precipitated as a liquid crystalline film assume a unique physical arrangement and share a common electronic structure. This introduces two significant effects. The overall absorption cross section of the liquid crystal to photons moving parallel to the axes of the molecules is expanded. In addition, because of the Pauli Exclusion Principle, the spectral absorption characteristic of the materialis broadened.
This spectral broadening is shown in Frame B for each of the chromophores of biological vision. There are four chromophores that account for all known biological vision. They are shown in Frame B along with their common molecular resonance peaking at 502 nm. While the chromophores are nominally equal in absolute isotropic absorption, the absolute absorption of the molecular absorption is considerably less intense than suggested by the normalization process in the figure. The wavelength of peak absorption of the four chromophores varies depending on the conjugation level of the molecule. In the figure, the conjugation level is described by indicating the location of carbon atom supporting the second oxygen atom using the notation of Karrer. As an example, the ultraviolet chromophore is listed as Rhodonine(11). Beginning in the ultraviolet, the spectral peaks shown correspond to a conjugation levels of two, three, four and five respectively. These molecular structures are available. The molecular absorption common to all of the materials in dilute solution is shown by the black line for reference.
Each of the above four chromophores is available in three chemical forms, depending on whether their chromogen was Vitamin A1, Vitamin A2 or Vitamin A3. [1.2.1]
Since the materials absorb anisotropically when in high concentration. The width of the individual absorption characteristics is determined by the conditions of solvation and the specific parameters of the measurement.
This frame employs a logarithmic vertical scale. It shows the normalized absorption characterisitic of the four chromophores of human vision in color. The half-amplitude width of these characteristics are shown for the in-vivo condition where more than 4000 monolayers of the liquid crystalline material are arranged on the surfaces of the disk in a typical Outer Segment.
The upper part of this frame shows the calculated spectral sensitivity (aka luminous efficiency function) of the human visual system under a variety of conditions using the theory of this work and the absorption characteristics of the Rhodonines in the liquid crystalline state described above.
Although the spectral response of the human eye has degenerated through evolution to the point where its sensitivity in the ultraviolet is minimal, the sensitivity of the retina itself is very high in the ultraviolet region. This sensitivity is illustrated by the heavy line including the area labeled aphakic on the left. If the lens of the eye is removed, the remaining visual system is found to be more sensitive in the ultraviolet than in the short wavelength region. The ultraviolet sensitivity approaches that in the mid wavelength region.
The absorption of the lens is the result of two mechanisms. The most important is the absorption due to the presence of aldehyde and alcohol ligands in the proteins of the lens. This absorption is significant and is shown by the closely spaced hatching on the left. As a result of this absorption, the overall spectral sensitivity of the eye is given by the heavy line enclosing the darkest area of the figure. Thre is also Rayleigh scattering in the physiological optics of the eye (including the lens) that increases with age (at about 0.5% per year). This absorption is shown by the less closely spaced hatching. The overall effect of this hatching is largely compensated for by the adaptation mechanism associated with the short wavelength photoreceptors of the eye.
The theoretical luminosity functions described in the figure reduce to the C.I.E. Standard Luminosity Functions when they are smoothed by a finite width spectral filter typical of the filters used in the spectrometers employed to describe the Standard Observor during the 1920's and 1930's, and are truncated below 400 by the absorption of the human lens group shown by the hatching. They are particularly accurate when compared to the Judd modification to the C.I.E. Standards proposed in the 1950's. [Section 17.3]
It is worth noting that the Purkinje Peak shown in the region of 580 nm is obtained under conditions of chromatic adaptation. It is the feature frequently assigned to the absorption of the long wavelength chromophore of vision by the psychophysical community. It is actually an artifact of inadequate chromatic adaptation when trying to isolate the long wavelength chromophore that actually peaks at 625 nm.
The peak labeled Brezold-Brucke ( near 487 nm) is due to chromatic adaptation causing the mid wavelength signaling channel to be reduced in amplitude to that of the short wavelength channel. It is predicted that there is a similar unnamed peak near 395 nm.
The above features are discussed in detail throughout the text of PROCESS IN BIOLOGICAL VISION. The individual luminousity functions are obtained by calculation within the visual/neural system. The mathematics involves a specific type of color signal differencing after spectral integration on a per channel basis. The architecture leading to the mathematical calculation are described in Chapter 11. Details of the mathematics are presented in Chapter 16. The fact that the luminous efficiency function is actually a single continuous function of a group of parameters is discussed in Chapter 17.
It is demonstrated that the retinenes do not exhibit the spectral or molecular properties associated with the chromophores of vision. They are in fact chromogens. The Rhodonines are the chromophores of vision. They do not rely upon any straining of the molecular structure nor do they employ isomeric changes to account for their performance. They generate an electronic signal using well understood quantum-mechanical principles to achive appropriate absorption and well understood quantum-electronic principles to create a free electron flux (a current) at the output of the photoreceptor cells.